-
home
Introduction and mission statement of our project.
-
people
Information about the PI, postdocs and students.
-
research
Description of our research, with brief introduction to our projects in computer vision, computational biology and neuroscience, and medical image computing.
-
publications
Journal and peer-review conference publications, and some abstracts.
-
collaborations
A list of our collaborations within the last five years. We acknowledge their contribution and their novelties in their fields.
-
sponsors
Federal organizations and private institutions that fund our research.
-
links
Some resources that we use and you may find useful.
-
contact
Contact information, including phone number, email, and location.
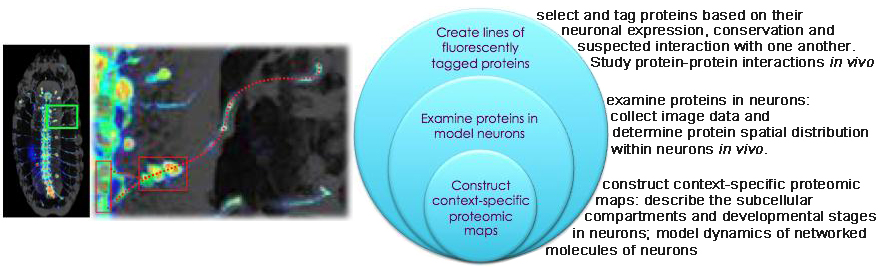
Computational Biology & Neuroscience
Modeling the Structure and Dynamics of Neuronal Circuits in the Drosophila larvae using Image Analytics (NSF CAREER Award)
Visit the project's website: http://neurovision.cs.iupui.edu/
The ability to adjust dynamically to attain stability in the face of widely ranging internal changes and external insults is a feature observed commonly in natural systems. The human brain, for example, has an amazing capacity to functionally recover from strokes that caused damages to local neuronal circuitries. Despite their scientific and social values, little is known about the principles of such highly adaptive systems. However, recent advances in imaging and computational technologies are practically ripe for visualizing and processing the small insect brain in its entirety, down to the level of individual synaptic connectivity.
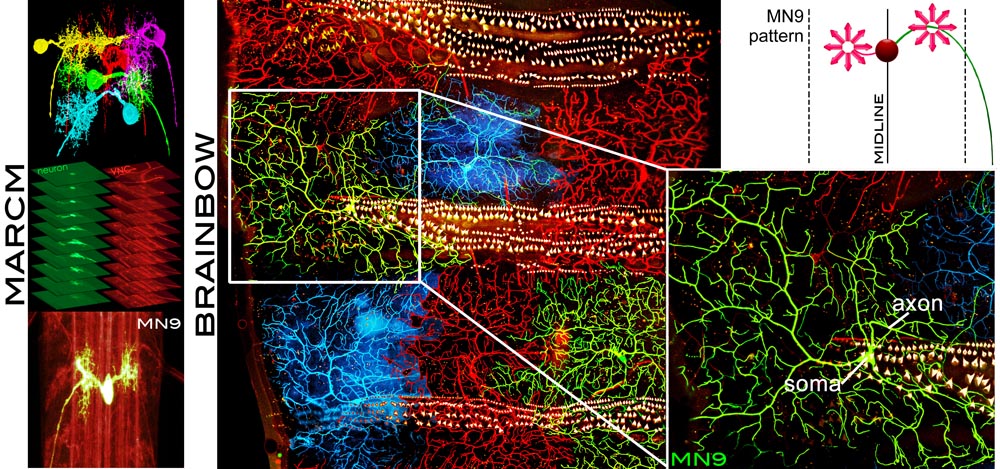
The objective of this project is the image-based computational modeling of how synaptic connectivity is established in vivo during brain development, a major question in neuroscience today. Using imagery acquired with state-of-the-art imaging techniques (Brainbow and MARCM) at two Drosophila neuroscience laboratories, the goal is to estimate and pattern the complete morphology, connectivity properties and structure dynamics of single neurons and neuronal circuits in the Drosophila larvae. This research effort aims at setting the principle for large-scale studies of more complex brains at single-cell resolution, and modeling adaptive responses of neuronal circuits to changes such as aging, disease and injury.
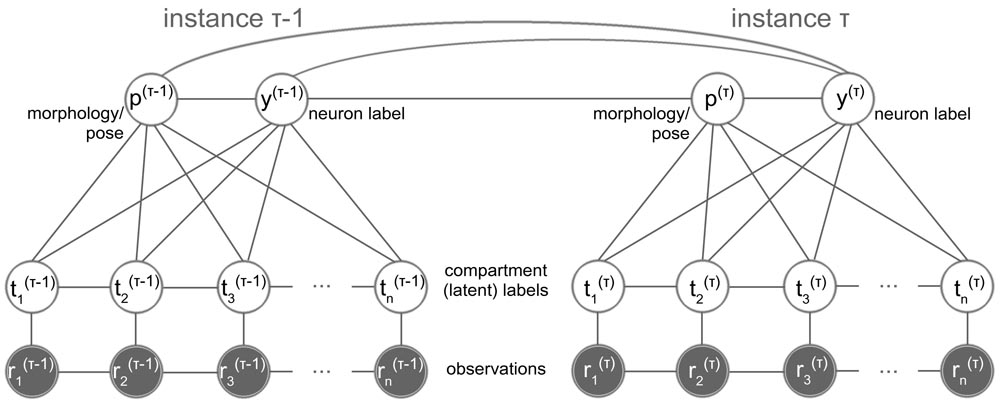
The overreaching hypothesis of this research is that the brain is a highly adaptive system defined by specific structure and dynamics, as a whole and at the single cell level. Although this is a fundamental hypothesis, it has been difficult to test using live animals, quantitatively through numerical modeling, or even qualitatively through observation. By bringing together leading-edge imaging technologies and computationally intensive image analytics, this project initiates the pursuit of what makes the brain function as a whole throughout life and continuously adapt to various changes such as aging, disease, drug treatment, and injury.
The research plan includes training a number of graduate, undergraduate and K12 students, integrating cutting-edge research in Computer Science and Computational Neuroscience with education.
Modeling the drosophila brain at single-cell resolution (NSF-DBI 1062405)
Visit the project's website: http://neurovision.cs.iupui.edu/
A collaborative work with Akira Chiba (Biol., U of Miami) and Michael Kim (Mol. & Cell. Pharmacol., U of Miami).
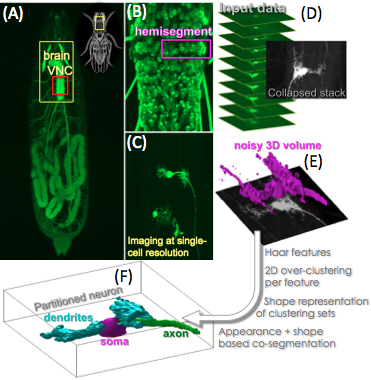
Modeling the neuronal circuitries at single-cell resolution in vivo can reveal in detail critical information about the Central Nervous System (CNS) and the synapses functionality, e.g., how motor neurons determine patterns in locomotory behaviors.
In the Drosophila larva, it has been shown that different subclasses of motor neurons follow specific morphological patterns with respect to the position, orientation and structure in the CNS [Kim et. al, 2009; Hoang and Chiba, 2001; Schmid et. al, 1999]. Thus, the study of the individual neurons' morphology, the detailed modeling and classification of the anatomical features of different neuron subclasses, can yield the automated identification of individual neurons in the CNS, which is a major step towards constructing the first neuronal/synaptic map of a model living brain.
In situ protein-protein interaction networks of neurons (NIH: 5RC2NS069488-02)
Visit the isPIN project: http://ispinproject.org/
A field of our research is proteomics, and its re-definition as a content-rich molecular bioinformatics.
Proteomics has been hailed as 'the next big thing' after genomics. It has progressed from cataloging the whole complement of proteins, or proteome, to charting their interactions, or interactome. Yet the major predicament in proteomics today is its paucity of in situ contexts.
Teams from the life and computational sciences, including our team,
launched an NIH-funded imaging-based survey of protein-protein interaction networks within neurons. Our
ultimate goal is the reconstruction of genome-wide protein-protein interaction networks within each and every
subcellular compartment of neurons at progressive steps of their development. This project is the first
systematic inspection of when and where each protein-protein interaction takes place in vivo.
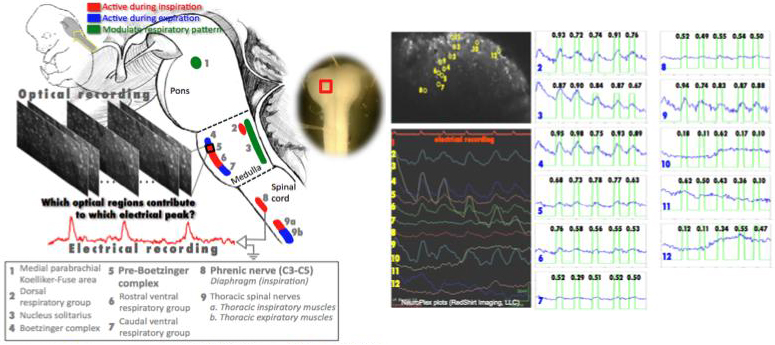
Associating the brainstem cell activity with the respiration rhythm
A collaborative work with K.J. Muller (U of Miami, USA), L.B. Cohen (Yale, USA), R. Homma (Yale, USA), J.G. Nicholls (SISSA, Italy), and J. Eugenin (USACH, Chile)
Optical recording of the activity of hundreds of individual neurons simultaneously within the functioning brain is now possible with calcium sensitive dyes. This offers a major advance over the limitations of single-unit recording with arrays of microelectrodes, or with functional MRI.
However, the analysis of optical activity to understand neuronal interactions and circuitry underlying physiological functions requires new computational approaches. Recently it has been possible to record optically from the distributed population of neurons in the brain stem generating the respiratory rhythm, breath by breath, using the compact brain stem and spinal cord preparation of the fetal mouse stained in vitro with [Ca2+] sensitive dye. The simultaneous electrical activity of phrenic motoneurons that innervate the diaphragm measures the timing of inspiratory breaths. In this project, fluorescence micrographs taken at 4-100Hz over 20-40sec (2-photon microscopy) have been analyzed with the simultaneously recorded electrical signal from the phrenic nerve.
We classify the brainstem regions into 'active' and 'inactive', based on whether they contribute or not to the individual electrical signal peaks. Initially we used a Conditional Random Field, using as observations the cross-correlations between the electrical recording and the individual intensity signals. However, this segmentation is based on global features (over the entire recording time), and therefore cannot reveal which brainstem regions are responsible for individual inspiration cycles (electrical signal peaks).
Motivated by our earlier work, and to compare optical and electrical signals locally, we
use the Continuous Wavelet Transform (CWT)-based Semblance analysis, to analyze signal content (similarity) over the whole frequency spectrum and varying frequency content over time. For the region classification, and to avoid the limitations of discriminative learning, we use a novel Generative Mixture Model -based clustering approach, in a possibilistic formulation.
C. elegans locomotory behavior classification
A collaborative work with Bianchi's (U of Miami) and Driscoll's (Rutgers) C. elegans labs
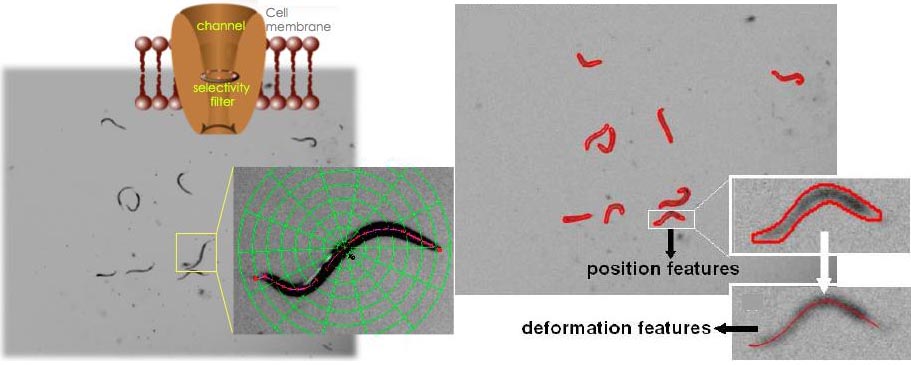
The nematode Caenorhabditis elegans (C. elegans) is a genetic model widely used to dissect conserved basic biological mechanisms of development and nervous system function. C. elegans locomotion is under complex neuronal regulation and is impacted by genetic and environmental factors, thus its analysis is expected to shed light on how genetic, environmental, and pathophysiological processes control behavior.
To date, computer-based approaches have been used for analysis of C. elegans locomotion, however, none of these is both high resolution and high throughput. We used computer vision methods to develop a novel automated approach for analyzing the C. elegans locomotion. Our method provides information on the position, trajectory, and body shape during locomotion and is designed to efficiently track multiple animals in cluttered images and under lighting variations.
We used this method to describe in detail C. elegans movement in
liquid for the first time and to analyze six unc-8, one mec-4 and
one odr-1 mutants. We reported features of nematode swimming
not previously noted and show that our method detects
differences in the swimming profile of mutants that appear at
first glance similar.